Laboratory Guide: Determination of Pesticide Residues According to AOAC 970.52, 985.22, and 970.53 (1990)
- Laboratory Guide: Determination of Pesticide Residues According to AOAC 970.52, 985.22, and 970.53 (1990)
Standard Operating Procedure (SOP)
1.0 Scope
This SOP applies to the detection and quantification of pesticide residues in fruits, vegetables, and plant-based matrices, following AOAC 970.52, 985.22, and 970.53.
2.0 Principle
Pesticides are extracted using solvents, purified by Florisil/silica cleanup, concentrated, and quantified via Gas Chromatography (GC) with selective detectors:
ECD (Electron Capture Detector): Organochlorines
NPD (Nitrogen Phosphorus Detector): Organophosphates and Organonitrogen
FPD (Flame Photometric Detector): Organophosphates
3.0 Responsibilities
Analyst: Conducts the procedure and maintains records.
Supervisor: Reviews QC, validates results.
QA Officer: Ensures compliance with accreditation standards.
4.0 Safety and Precautions
Checklist before starting work:
☐ Lab coat, nitrile gloves, goggles
☐ Certified fume hood operational
☐ MSDS for solvents available
☐ Waste containers labeled (halogenated vs non-halogenated)⚠️ Hazards:
Solvents: Flammable and toxic → avoid ignition sources.
Standards: Extremely toxic → handle only with calibrated pipettes.
Florisil dust: Respiratory hazard → wear mask if handling dry powder.
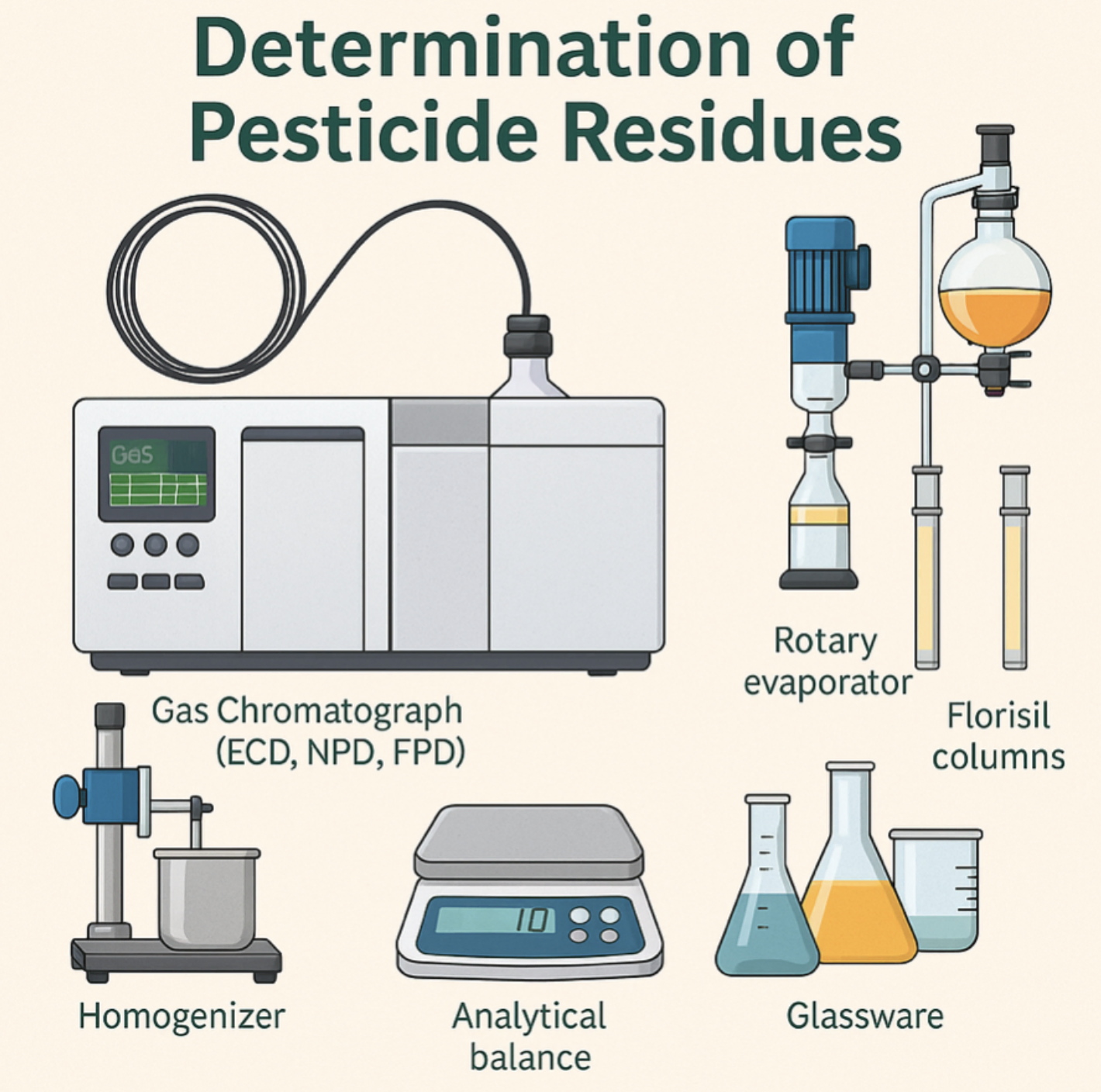
5.0 Equipment and Apparatus
Gas Chromatograph with ECD, NPD, FPD
Capillary columns DB-5 or DB-1701 (30 m x 0.25 mm x 0.25 μm)
Homogenizer (stainless steel jar)
Analytical balance (±0.1 mg)
Rotary evaporator with water bath ≤40 °C
Kuderna-Danish (K-D) concentrator tubes with Snyder column
Florisil glass columns with Teflon stopcock
Volumetric flasks, pipettes, centrifuge (optional)
6.0 Reagents and Standards
Solvents (residue grade): Acetonitrile, Petroleum Ether, Hexane, Acetone, Dichloromethane, Ethyl Acetate
Sodium Sulfate (anhydrous, pre-baked 600 °C, stored sealed)
Florisil (60–100 mesh, activated 675 °C, deactivated 1–2% water)
Certified pesticide standards (DDT, Malathion, Lindane, Carbaryl, etc.)
Calibration standards (multi-level: 0.01–0.5 µg/mL)
7.0 Procedure
7.1 Sample Preparation
Collect representative sample (50–100 g).
Homogenize using blender/homogenizer until uniform.
Record sample weight in logbook.
7.2 Extraction
AOAC 970.52 & 970.53 (Organochlorines, Organophosphates):
Add 200 mL acetonitrile to 50 g sample.
Blend 2–3 min.
Filter homogenate into suction flask.
Transfer 100 mL extract to separatory funnel.
Add 100 mL petroleum ether → shake.
Add 500 mL sodium sulfate solution (10%) → shake.
Discard aqueous layer, retain petroleum ether layer.
Wash extract twice with 100 mL sodium sulfate solution.
Pass through sodium sulfate drying bed.
AOAC 985.22 (Organonitrogen pesticides):
Extract with dichloromethane.
Partition into acetonitrile-saturated hexane (removes fats).
Back-extract into dichloromethane.
7.3 Cleanup (Florisil Column Chromatography)
Pack glass column:
Glass wool → 2 cm Na₂SO₄ → 10–15 g Florisil → 2 cm Na₂SO₄
Condition with 50 mL petroleum ether.
Load concentrated extract.
Elute sequentially:
Fraction 1: 200 mL (6% ether/petroleum ether) → HCB, Lindane
Fraction 2: 200 mL (15%) → Aldrin, Dieldrin
Fraction 3: 200 mL (50%) → Malathion, Parathion
7.4 Concentration
K-D concentrator + Snyder column in 40 °C bath.
Reduce volume to 5–10 mL.
Final concentration under N₂ → 1–2 mL (hexane).
7.5 GC Analysis
Carrier gas: Helium/Hydrogen.
Injection: 0.5–1 µL, splitless.
Injector temp: 250 °C.
Oven: 80 °C (1 min) → ramp 10–15 °C/min → 280 °C (hold 10 min).
Detector temps: ECD 320 °C; NPD 280 °C; FPD 250 °C.
Run calibration standards and samples.
Confirm retention times vs standards.
8.0 Quality Control (QC)
QC Parameter Frequency Acceptance Criteria Method Blank 1/batch No pesticide detected LCS (spiked sample) 1/batch Recovery 70–120% Duplicate sample 1/batch RSD ≤20% Calibration check Every 10–12 injections ±20% of initial response 9.0 Calculations
Residue (mg/kg or ppm) = (C × V) / (W × R)
Where:
C = Analyte concentration in extract (µg/mL)
V = Final extract volume (mL)
W = Sample weight equivalent (g)
R = Recovery correction (if applied, else R=1)
10.0 Records
Sample logbook entries
Extraction sheets
GC run sheets with chromatograms
QC summary report
11.0 Method Limitations
Historical AOAC methods → limited for modern pesticides.
Not confirmatory (requires GC-MS/MS).
Possible matrix effects.
- For more information:
? : Contact Us